New Physician Insights Reveal Building Patient Trust and Education Will Be Crucial for Successful Vaccine Rollout
New York – August 20, 2020 – A global survey of nearly 2,000 physicians across 31 countries conducted from August 8-11, reveals nearly half of physicians surveyed see significant barriers to COVID-19 vaccine adoption, both for themselves and for their adult patients.
While the effectiveness and safety of vaccines are matters of paramount importance to doctors and their patients, 48 percent of U.S. physicians surveyed reveal their patients don’t believe COVID-19 is a serious concern for them – making this the third most significant barrier to the potential adoption of a vaccine. The survey, called the COVID-19 Real Time Barometer, was conducted by leading physician insights company, Sermo, and is the 13th survey in an ongoing observation study designed to take a comprehensive look at the impact of COVID-19 through the lens of the global physician community.
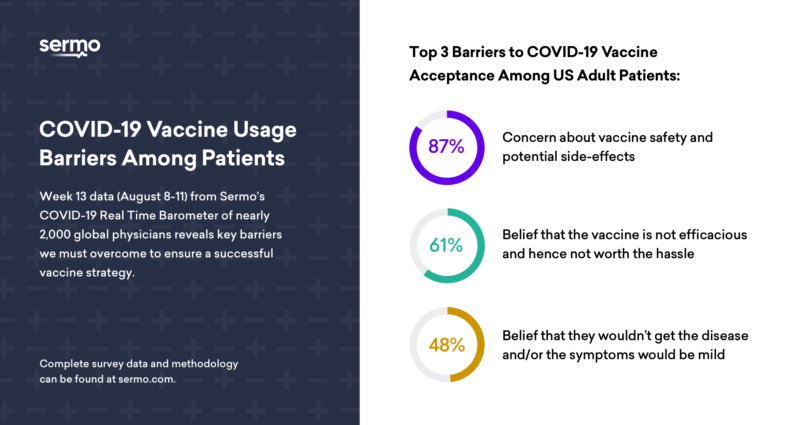
When asked what physicians think will be the top three barriers to acceptance of a COVID-19 vaccine among adult patients in the U.S.:
- 87 percent believe concern over vaccine safety and potential side-effects will be a significant barrier to some of their adult patients receiving vaccination
- 61 percent think that some of their patients will have concerns about the efficacy of a vaccine and believe it may not to be worth the hassle
- 48 percent said some of their patients believed that they wouldn’t get the disease and/or the symptoms wouldn’t be too bad if they did
Balancing benefit versus risk is always a critical factor in a physician’s decision to prescribe. However, in the case of a potential COVID-19 vaccine, the survey findings suggest that this becomes even more important as it is compounded by the persistent belief among many patients that vaccines in general aren’t safe or that they feel impervious to infection.
“As a family physician, I’ve been treating patients with vaccines for over 40 years, and I often have to contend with patients’ trepidations, concerns and misinformation about the safety of vaccines,” said Roger Hofford, M.D., Sermo member and associate professor, Virginia Tech Carilion School of Medicine and clinical professor of Family Medicine and Population Health at Virginia Commonwealth University. “These same issues will likely also have an impact on the general acceptance of a potential COVID-19 vaccine. Not only do we anticipate our patients will have safety concerns, many of our patients don’t feel they are at a significant enough risk to warrant a vaccine, adding one more hurdle to vaccine acceptance.”
“Physicians want to prescribe with confidence, knowing that a treatment does what it is supposed to do with minimal side effects and without long-term consequences,” Dr. Hofford added. “With the FDA bar for entry requiring 50 percent efficacy, we may see a resistance from both physicians and patients – with patient misperceptions and preexisting fears about vaccines combined with a false sense of security contributing to a low rate of vaccination.”
In order to understand the drivers behind vaccine adoption among physicians, the survey applied a common methodology used in healthcare research: a discrete choice experiment. This methodology elicits preferences for vaccine features (attributes) through multiple questions that ask physicians to select their vaccines they would recommend to specific patient types among a set of vaccine profiles. The hypothetical profiles combine features for which can be used to define actual vaccines when made available. The features are:
- Level of potential adverse events
- Protection rate
- Immunity Duration
- Dosing regimen
- Platform technology
- Testing history
- Manufacturer type
Based on anecdotal information about vaccines being tested and proposed governmental guidelines for requirements to approve a COVID-19 vaccine, the analysis established a “likely vaccine profile”:
- Level of potential adverse events: moderate events
- Protection rate: 70%
- Immunity Duration: 12 months
- Dosing regimen: 2 dose regimen (e.g., 1 dose + booster at 1 month)
- Platform technology: Novel platform (e.g., DNA or RNA vaccines)
- Testing history: Established safety data set 9 months since first patient (incl. 6 months of 30,000 individuals ages 18+)
- Manufacturer type: Established pharmaceutical company
When assessing vaccine profiles and physician willingness to recommend, the survey found that physicians will be cautious in their recommendations but are more inclined to recommend to at-risk patients. For example, only 70 percent of physicians indicated they would recommend the “likely vaccine profile” to children due in large part to concerns over adverse events, whereas 84 percent said they would recommend the “likely vaccine profile” to older and at-risk patients (adults>65 and at-risk adults with comordities and occupational).
That willingness to prescribe a vaccine to older, at-risk patients (adults>65 and at-risk adults with comordities and occupational) increased only slightly among physicians (88%) when presented with the best case vaccine profile showing longer, 24-month protection and mild potential adverse events. Similarly, the willingness to recommend to children also increased among physicians (78%) with the best case vaccine profile due to its improved safety profile.
Physicians will be paying close attention to the potential for adverse events and moderate attention to the protection rate as they trade off the benefits of the vaccine for their patients. This is particularly true for less at-risk populations: children, adolescents and adults under 65 with no pre-existing conditions or comorbidities. Even for at-risk patients, for whom willingness to prescribe any approved vaccine is high (always a majority), we find lower willingness as the threat of adverse events becomes more severe and protection rate is at 50%.
“Since the start of the pandemic, we’ve all been overwhelmed with COVID-19 information and the constant change it has been invoking in our daily lives,” said Peter Kirk, Sermo CEO. “When an FDA-approved vaccine becomes available, ensuring that healthcare providers are armed with tools to build trust and help educate their patients on vaccine safety and efficacy will be crucial to providing our communities with the information they need to make the most informed decisions to protect themselves, their families and their communities.”
Complete survey data and methodology can be found at sermo.com.
About the Real Time Barometer
The Real Time Barometer is an observational study of the impact of the COVID-19 outbreak as reported by physicians with firsthand experience treating COVID-19 patients. Each week, thousands of physicians who are active participants in the Sermo community provide insights on topics regarding the global health crisis. To date, Sermo has conducted 55,280 interviews with doctors in 31 countries, including the United States, Canada, United Kingdom, France, Brazil, Russia, China, Japan, and Australia.
About Sermo
Sermo is the largest healthcare data collection company and social platform for physicians, reaching 1.3MM healthcare professionals across 150 countries. The platform enables doctors to anonymously talk real-world medicine, review treatment options via our proprietary Drug Ratings platform, collectively solve patient cases, and participate in medical market research. For more information, visit sermo.com.