Impact of abortion case on physicians The US Supreme Court is taking on a key…


Impact of abortion case on physicians The US Supreme Court is taking on a key…

Wondering if Sermo is legit? Discover how 1M+ physicians use the verified community to collaborate,…
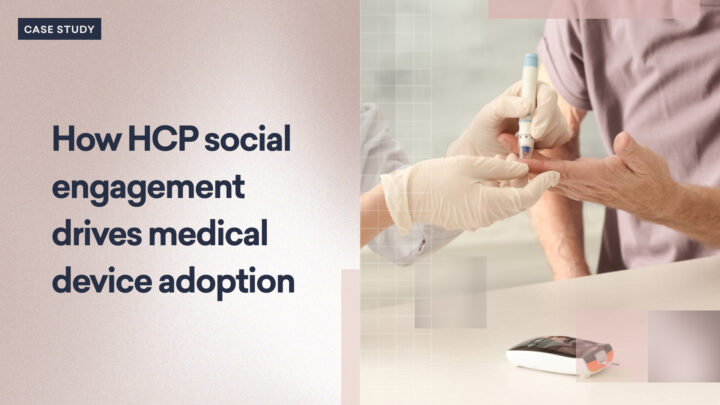
Marketing a new medical device in today’s competitive healthcare landscape demands not only innovative strategies…
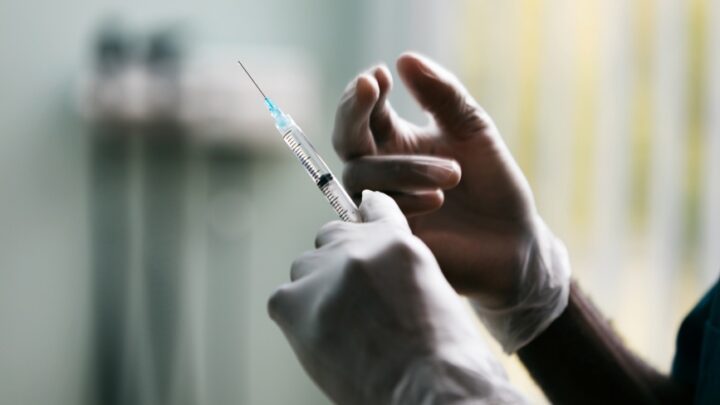
As the rates of seasonal and routine vaccinations decline, reports of increasing cases of preventable…

Medical congresses are a hub of innovation, connection, and opportunity. They’re where groundbreaking research is…

As the number of nurse practitioners (NPs) skyrockets, their impact on healthcare has come into…

The medical landscape is evolving, with HCPs stepping into the spotlight as influential voices from…

Over 47 million Americans have a “silent” build-up of amyloid plaques—high levels of amyloid plaques…

The Centers for Disease Control and Prevention (CDC) recommends universal screening to ensure every patient…

The digitalization of healthcare has boosted AI adoption. Marketers are now leading the way in…

In the rapidly evolving world of healthcare communications, hyper-targeted, data-driven approaches are the new standard…

With the rise of omnichannel HCP marketing, nearly 8 in 10 physicians say digital resources…

The heartbeat of the healthcare industry lies in the insights and experiences of its leaders. …